
The first law, also known as Law of Conservation of Energy, states that energy cannot be created or destroyed in an isolated system.

What is the First and Second Law of Thermodynamics? Differences in temperature, pressure, and density tend to even out horizontally after a while. What is the second law of thermodynamics for dummies?Īpril 2012) The second law of thermodynamics says that when energy changes from one form to another form, or matter moves freely, entropy (disorder) in a closed system increases. for all types of working fluids used in the engines. This law is applicable to all types of heat engine cycles including Otto, Diesel, etc. What are the applications of the second law of thermodynamics? 1) According to the law, heat always flows from a body at a higher temperature to a body at the lower temperature.

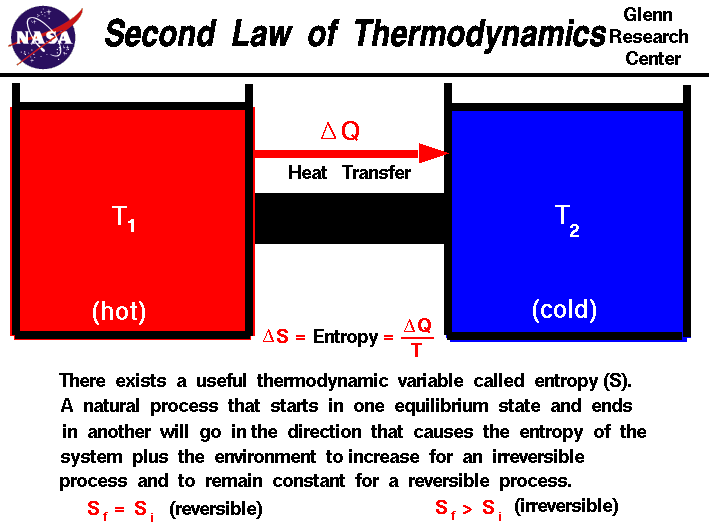
The second law also states that the changes in the entropy in the universe can never be negative. You might be interested: Law and order where to watch What is the application of second law of thermodynamics? The Second Law of Thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time. … This means that the entropy of the universe is constantly increasing. Entropy is the measure of disorder: the higher the disorder, the higher the entropy of the system. The most popular concept related to entropy is the idea of disorder. What does the law of entropy tell us?Įntropy is one of the consequences of the second law of thermodynamics. However, all living things maintain a highly ordered, low entropy structure. We can view the entire universe as an isolated system, leading to the conclusion that the entropy of the universe is tending to a maximum. Does life violate the 2nd law of thermodynamics? … The Second Law of Thermodynamics states that “in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.” This is also commonly referred to as entropy. What does the second law of thermodynamics say?Įnergy is the ability to bring about change or to do work. Entropy is zero in a reversible process it increases in an irreversible process. Another form of the second law of thermodynamics states that the total entropy of a system either increases or remains constant it never decreases. How does entropy relate to the second law of thermodynamics?Įntropy is the loss of energy available to do work.


 0 kommentar(er)
0 kommentar(er)
